Robust artificial interphases constructed by a versatile protein-based binder for high-voltage Na-ion battery cathodes
 © 2022 Wiley-VCH GmbH
© 2022 Wiley-VCH GmbHAbstract
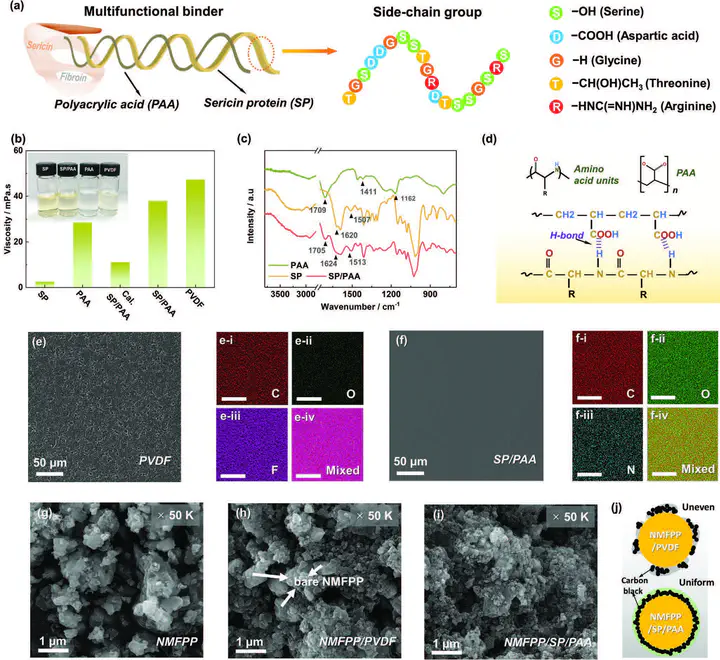
The multiple issues of unstable electrode/electrolyte interphases, sluggish reaction kinetics, and transition-metal (TM) dissolution have long greatly affected the rate and cycling performance of cathode materials for Na-ion batteries. Herein, a multifunctional protein-based binder, sericin protein/poly(acrylic acid) (SP/PAA), is developed, which shows intriguing physiochemical properties to address these issues. The highly hydrophilic nature and strong H-bond interaction between crosslinking SP and PAA leads to a uniform coating of the binder layer, which serves as an artificial interphase on the high-voltage Na4Mn2Fe(PO4)2P2O7 cathode material (NMFPP). Through systematic experiments and theoretical calculations, it is shown that the SP/PAA binder is electrochemically stable at high voltages and possesses increased ionic conductivity due to the interaction between sericin and electrolyte anion ClO4−, which can provide additional sodium-migration paths with greatly reduced energy barriers. Besides, the strong interaction force between the binder and the NMFPP can effectively protect the cathode from electrolyte corrosion, suppress Mn-dissolution, stabilize crystal structure, and ensure electrode integrity during cycling. Benefiting from these merits, the SP/PAA-based NMFPP electrode displays enhanced rate and cycling performance. Of note, the universality of the SP/PAA binder is further confirmed on Na3V2(PO4)2F3. It is believed that the versatile protein-based binder is enlightening for the development of high-performance batteries.