Reactivity-Tunable Fluorescent Platform for Selective and Biocompatible Modification of Cysteine or Lysine
Abstract
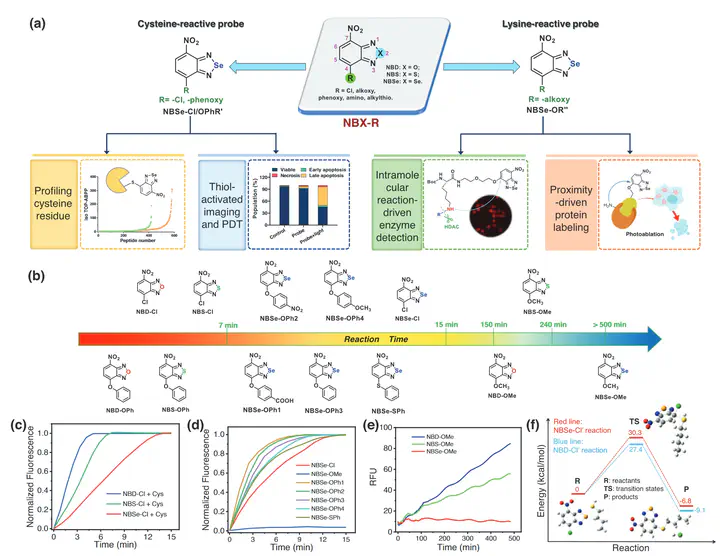
Chemoselective modification of specific residues within a given protein poses a significant challenge, as the microenvironment of amino acid residues in proteins is variable. Developing a universal molecular platform with tunable chemical warheads can provide powerful tools for precisely labeling specific amino acids in proteins. Cysteine and lysine are hot targets for chemoselective modification, but current cysteine/lysine-selective warheads face challenges due to cross-reactivity and unstable reaction products. In this study, a versatile fluorescent platform is developed for highly selective modification of cysteine/lysine under biocompatible conditions. Chloro- or phenoxy-substituted NBSe derivatives effectively labeled cysteine residues in the cellular proteome with high specificity. This finding also led to the development of phenoxy-NBSe phototheragnostic for the diagnosis and activatable photodynamic therapy of GSH-overexpressed cancer cells. Conversely, alkoxy-NBSe derivatives are engineered to selectively react with lysine residues in the cellular environment, exhibiting excellent anti-interfering ability against thiols. Leveraging a proximity-driven approach, alkoxy-NBSe probes are successfully designed to demonstrate their utility in bioimaging of lysine deacetylase activity. This study also achieves integrating a small photosensitizer into lysine residues of proteins in a regioselective manner, achieving photoablation of cancer cells activated by overexpressed proteins.
Highlights:
In summary, we have demonstrated that NBSe derivatives possess unique reactivity and can be rationally developed as highly efficient fluorescent warheads for selective labeling of cysteines or lysines under biocompatible conditions. Chloro or phenoxy-substituted NBSe derivatives exhibit high specificity toward cysteines in proteome labeling, providing new opportunities for chemical proteomic profiling and covalent-drug design. On the other hand, alkoxy-NBSe, a latent electrophile, can be designed to selectively react with lysines in a protein in regioselective manner using proximity-driven strategy. In addition, NBSe derivatives exhibit unique photophysical properties, and can serve as imaging fluorophores as well as small-sized photosensitizers. Although the current probes have not yet been effective for in vivo PDT experiments, we can further expand the conjugation system to shift absorption to longer wavelengths or extend the strategy to design activatable PDT triggered by cancer-overexpressed proteins. We envision that our study will provide new insight into designing selective warheads for proteinogenic amino acid modification and add useful tools for the development of protein-based therapeutics.
Supplementary Information can be accessed here.