Controllable cleavage of C–N bond-based fluorescent and photoacoustic dual-modal probes for the detection of H2S in living mice
 Copyright © 2021 American Chemical Society
Copyright © 2021 American Chemical SocietyAbstract
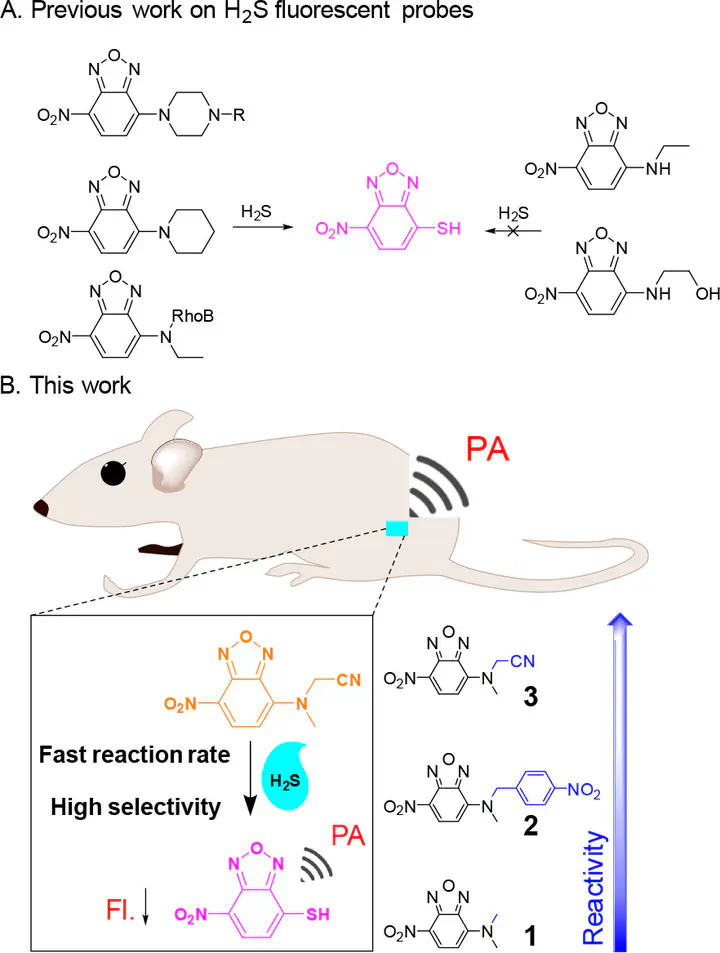
Hydrogen sulfide (H2S) has been recognized to influence a wide range of physiological and pathological processes. Its underlying molecular events, however, are still poorly understood. An activatable H2S probe for photoacoustic (PA) imaging is desirable to further explore the role of H2S in vivo. Nevertheless, only a few activatable PA probes for H2S detection have been reported. In particular, examples of dual-modal H2S probes with the combined advantages of fluorescence (high sensitivity and resolution) and PA imaging (deep penetration) are very rare. Herein the controllable cleavage of the C–N bond in nitrobenzoxadiazole (NBD) amine derivatives by H2S is presented for the first time. The cleavage reactivity was found to be accelerated by the introduction of an electron-withdrawing group. Through this strategy, a series of fluorescent and PA dual-modal probes (1–3) were developed for H2S detection. Among them, probe 3 shows a high fluorescence on–off response rate (k2 = 4.04 M–1 s–1) and excellent selectivity for H2S over other biothiols. Moreover, probe 3 can also work as an activatable PA H2S probe because of the significant shift of its absorption peak from 468 to 532 nm in the H2S reaction. Importantly, probe 3 demonstrates its capability for fluorescence and PA imaging of H2S in living cells and mice. These results indicate that the controllable cleavage of the C–N bond can serve as an efficient strategy for designing fluorescent and PA dual-modal H2S probes.
Supplementary Information can be accessed here