Affinity-based protein profiling reveals cellular targets of photoreactive anticancer inhibitors
 Copyright © 2019 American Chemical Society
Copyright © 2019 American Chemical SocietyAbstract
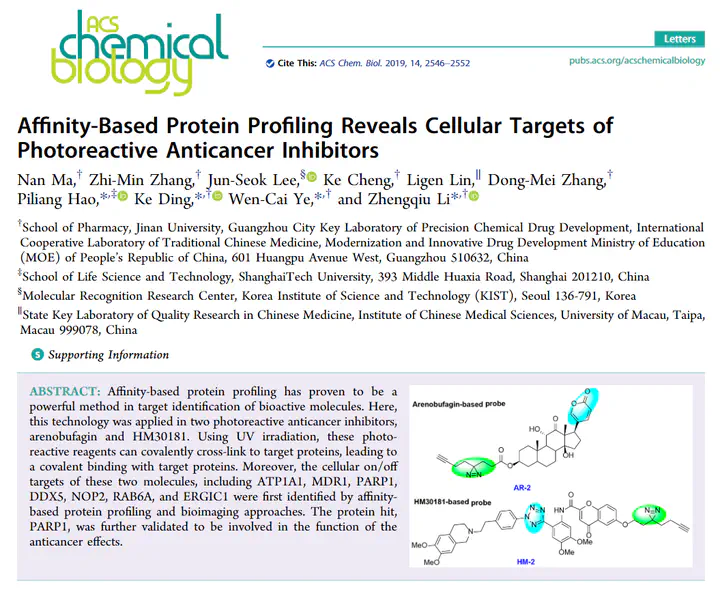
Affinity-based protein profiling has proven to be a powerful method in target identification of bioactive molecules. Here, this technology was applied in two photoreactive anticancer inhibitors, arenobufagin and HM30181. Using UV irradiation, these photoreactive reagents can covalently cross-link to target proteins, leading to a covalent binding with target proteins. Moreover, the cellular on/off targets of these two molecules, including ATP1A1, MDR1, PARP1, DDX5, NOP2, RAB6A, and ERGIC1 were first identified by affinity-based protein profiling and bioimaging approaches. The protein hit, PARP1, was further validated to be involved in the function of the anticancer effects.
Type
Publication
In ACS Chemical Biology