Self-assembled nano-photosensitizer for targeted, activatable, and biosafe cancer phototheranostics
 © 2022 Elsevier Ltd. All rights reserved.
© 2022 Elsevier Ltd. All rights reserved.Abstract
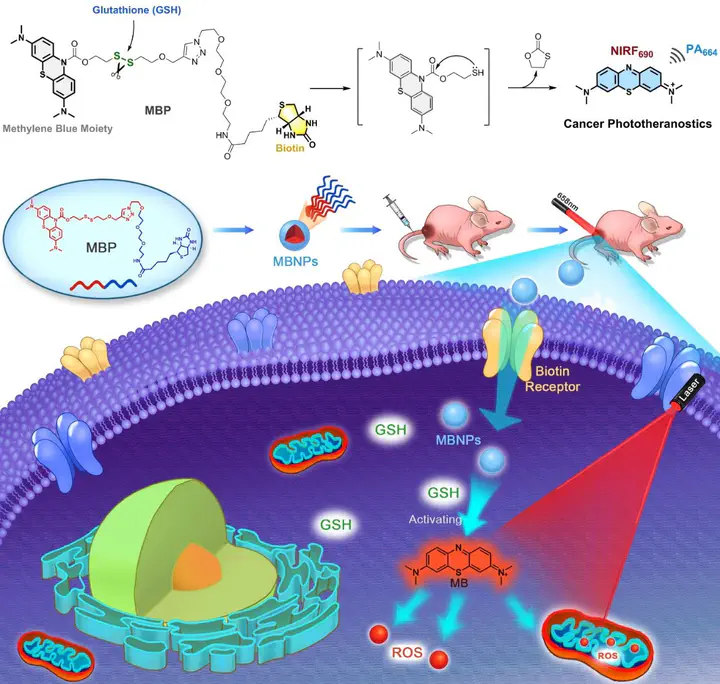
Cancer treatment currently still faces crucial challenges in therapeutic effectiveness, precision, and complexity. Photodynamic therapy (PDT) as a non-invasive tactic has earned widespread popularity for its excellent therapeutic output, flexibility, and restrained toxicity. Nonetheless, drawbacks, including low efficiency, poor cancer specificity, and limited therapeutic depth, remain considerable during the cancer treatment. Although great effort has been made to improve the performance, the overall efficiency and biosafety are still ambiguous and unable to meet urgent clinical needs. Herein, this study integrates merits from previous PDT strategies and develops a cancer-targeting, activatable, biosafe photosensitizer. Owing to excellent self-assembly ability, this photosensitizer can be conveniently prepared as multifunctional nano-photosensitizers, namely MBNPs, and applied to in vivo cancer phototheranostics in “all-in-one” mode. This study successfully verifies the mechanism of MBNPs, then deploys them to cell-based and in vivo cancer PDT. Based on the unique cancer microenvironment, MBNPs achieve precise distribution, accumulation, and activation towards the tumor, releasing methylene blue as a potent photosensitizer for phototherapy. The PDT outcome demonstrates MBNPs’ superior cancer specificity, remarkable PDT efficacy, and negligible toxicity. Meanwhile, in vivo NIR fluorescence and photoacoustic imaging have been utilized to guide the PDT treatment synergistically. Additionally, the biosafety of the MBNPs-based PDT treatment is ensured, thus providing potential for future clinical studies.
Highlights:
1. A GSH-triggered, activatable, and cancer-targeting probe is developed based on the rational design of methylene blue. This multifunctional probe can self-assemble into nano-photosensitizer, MBNPs.
2. In vitro and cellular experiments demonstrate the mechanism and superior therapeutic efficacy/precision of MBNPs.
3. MBNPs are applied to in vivo cancer PDT experiments, demonstrating good biosafety and remarkable efficacy/precision in tumor ablation.
4. The “all-in-one” tactic achieves fluorescence/photoacoustic guided cancer phototheranostics with single small molecules.
Supplementary Information can be accessed here.