Black phosphorus nanosheets inhibit glioblastoma cell migration and invasion through modulation of WNT/β-catenin and NOTCH signaling pathways
 © 2024 Elsevier Ltd. All rights reserved.
© 2024 Elsevier Ltd. All rights reserved.Abstract
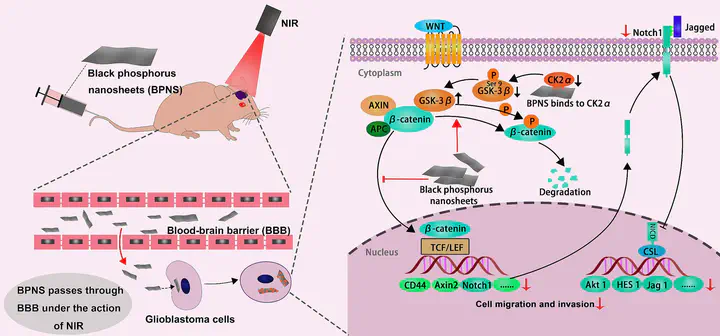
Black phosphorus nanosheet (BPNS) has recently demonstrated intrinsic anti-tumor bioactivity, but its underlying molecular mechanism remains unclear, limiting its potential applications in biomedicine. In this study, we investigated the impact of BPNS on glioblastoma cells and observed a significant dose-dependent inhibition of invasion and migration. RNA sequencing analysis revealed downregulation of genes associated with the WNT/β-catenin and NOTCH signaling pathways, both linked to invasion and migration. Mechanistically, BPNS directly binds to CSNK2A2, reducing its kinase activity, which indirectly enhances GSK-3β kinase activity. As a result, GSK-3β increases the phosphorylation level of β-catenin, leading to its degradation and subsequent inhibition of downstream molecules in the WNT/β-catenin signaling pathway. Our study uncovers the inherent biological activity of BPNS in hindering glioblastoma invasion and migration and sheds light on the molecular mechanisms, offering new directions for the nanomaterial’s biomedical applications.
Highlights:
• Black phosphorus nanosheet (BPNS) possesses new biological activity.
• BPNS effectively inhibits the invasion and migration of glioblastoma (GBM).
• The WNT/β-catenin signaling pathway is the key pathway for BPNS‘s bioactivity.
• BPNS directly binds to CSNK2A2 leading to the inhibition of this signaling pathway.
• BPNS holds promise as a potential therapeutic strategy for GBM.
Supplementary Information can be accessed here.